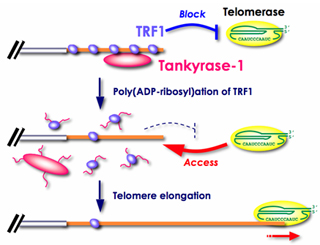
Tankyrase, a telomeric poly(ADP-ribose) polymerase (PARP), "acts as a positive regulator of telomere enlongation in vivo, apparently by inhibiting TRF1 [Index]. In general, PARP catalyzes the formation of poly(ADP-ribose) onto a protein acceptor using NAD+ as a substrate in response to DNA damage.
Long-term overexpression of tankyrase 1 in telomerase-positive human cells results in a gradual and progressive enlongation of telomeres. See "Overexpression of tankyrase 1 in telomerase-positive cells releases TRF1 from telomeres, resulting in telomere elongation." [2004], and H. Seimiya (2006),
The telomeric PARP, tankyrases, as targets for cancer therapy, British Journal of Cancer, 2006, 94, 341-345 [Figure 2].
Tankyrase 1 uses NAD as a stubstrate in poly(ADP-ribosylation) of TRF1, so NAD supplements [Images] may be useful when applying Tankyrase 1 to encourage hTERT transcription to activate telomerase.
Tankyrase 1 has more than 1 function in the cell. "Tankyrase 1 is a novel signaling target of mitogen-activated protein kinase (MAPK); it is stoichiometrically phosphorylated upon insulin stimulation. Phosphorylation enhances the poly(ADP-ribose) polymerase activity of tankyrase 1..." (Nai-Wen Chi and Harvey F. Lodish, 2000).
Phosphorylation allows tankyrase 1 to transduce MAPK signaling into poly(ADP-ribosyl)ation of effector proteins such as TRF1.
See upregulating tankyrase 1 mRNA transcription [Papers]. "TRF1 is a specific negative regulator of telomere length and its poly(ADP-ribosylation) by tankyrase 1 leads to the loss of telomere association and subsequent degradation by the ubiquitin/proteasome pathway (Chang et al. 2003, Smith et al. 1998)." (W.N.Keith and A.E.Bilsland, Targeting Telomerase: Therapeutic Options for Cancer Treatment, in K. Lenhard Rudolph, 2008, p.269.)
Perhaps adding an extra gene for tankyrase 1 with Zinc Finger Nuclease technology would be life-extending, if it did not much encourage carcinogenesis. Alternatively, tankyrase 1 plasmid expression vectors may be useful. See promoting tankyrase transcription. Evidently, going for a strong insulin spike after a bodybuilding workout (by using a specially formulated post-workout shake) can phosphorylate tankyrase, improving its performance as a telomerase activator. Furthermore, insulin itself may have this effect.
Gymnema Sylvestre (400-500 mg with a postworkout shake < 30 min after exercise) stimulates insulin secretion. Dextrose may be taken at 25-50 grams to spike insulin, along with whey protein or whey hydrolysates after a bodybuilding workout. Insulin secretion may also be improved by Fenugreek seeds, Fenugreek Extract [Images], or 4-hydroxyisoleucine [Images]. Tankyrase action on TRF1 can probably be promoted using Gymnema Sylvestre [Images] to produce insulin, phosphorylating Tankyrase 1 to increase its telomerase activation activity.
Other compounds besides niacinamide might be used to improve NAD substrate levels for tankyrase 1 reactions with TRF1, and targeted phosphorylation of tankyrase 1 using substances besides insulin may be envisioned, perhaps vanadyl sulphate.
See also Cook, B. D., J. N. Dynek, W. Chang, G. Shostak, and S. Smith (2002), Role for the related poly(ADP-Ribose) polymerases tankyrase 1 and 2 at human telomeres, Mol. Cell. Biol, 22:332-342. Also of revelance is Ye J.Z., and de Lange, T. (2004), TIN2 is a tankyrase 1 PARP modulator in the TRF1 telomere length control complex, Nature Genetics, 36, 618-623. See also Nai-Wen Chi and Harvey F. Lodish (2000), Tankyrase Is a Golgi-associated Mitogen-activated Protein Kinase Substrate That Interacts with IRAP in GLUT4 Vesicles, Journal of Biological Chemistry, December 8, 2000, 275, 38437-38444. This is a reference for the phosphorylation of tankyrase 1 resulting in the upregulation of its activity. "In insulin-stimulated adipocytes, tankyrase 1 is phosphorylated at serine residues by the mitogen-activated protein kinase pathway." - After H. Seimiya, op.cit., referencing (Chi and Lodish, 2000), op.cit. Note that "...Tankyrase is quantitatively phosphorylated on certain serine residues by MAPK upon stimulation with insulin, PDGF, and EGF." (Chi and Lodish, 2000).
The phosphorylation of tankyrase 1 enhances its poly(ADP-ribo)sylation activity on TRF1 telomere loop closure protein, allowing t-loops to open for access by the telomerase holoenzyme. For application of tankyrase wisdom to cancer, see Hiroyuki Seimiya, Yukiko Muramatsu, Tomokazu Ohishi, and Takashi Tsuruo (2005), Tankyrase 1 as a target for telomere-directed molecular cancer therapeutics, Cancer Cell, Vol.7, issue 1, January 2005, pp.25-37. Also see Susan Smith and Titia de Lange (2000), Tankyrase promotes telomere enlongation in human cells, Current Biology, vol 10, no. 20, 1299-1302 with Susan Smith and Titia de Lange, TRF1 Binding Protein, Methods of Use Thereof, US Patent 6,277,613 B1. "Tankyrase does not contain a nuclear localization signal (NLS) and transfected tankyrase is excluded from the nucleus unless it is co-transfected with TRF1.... (An NLS can be added to its amino terminus to produce nuclear-localized tankyrase (FN-tankyrase))... Tankyrase releases TRF1 from telomeres in a reaction that depends on its PARP domain".
Doxycyclin